Introduction: The anti-CD38 antibody isatuximab (Isa) is approved in various countries with pomalidomide-dexamethasone for relapsed/refractory multiple myeloma (RRMM) patients (pts) with ≥2 prior therapies including lenalidomide and a proteasome inhibitor, based on the ICARIA-MM study, and with carfilzomib-dexamethasone for RRMM pts with ≥1 prior therapy, based on the IKEMA study. To enhance convenience of intravenous (IV) Isa administration, a shorter IV infusion over 30 minutes (30-min) was assessed in pts with NDMM not eligible/with no immediate intent for autologous stem cell transplantation (ASCT) still on maintenance therapy in a Phase 1b trial (NCT02513186). Results previously reported from this study showed efficacy of treatment with Isa in combination with bortezomib-cyclophosphamide-dexamethasone (VCd) or bortezomib-lenalidomide-dexamethasone (VRd), with manageable safety profiles. The rates of very good partial response or better and of minimal residual disease negativity (at 10 -5 sensitivity) were 80% and 53.3% with Isa-VCd, and 92.9% and 50.7%, respectively, with Isa-VRd [Ocio EM et al. HemaSphere 2023;7(2):e829; Ocio EM et al. Leukemia 2023;37(7):1521-1529]. Preliminary results with the new 30-min Isa administration method are presented here.
Methods: Pts still receiving maintenance treatment were to be switched to the 30-min infusion with Isa at 10 mg/kg diluted in a 250 mL infusion bag of 0.9% sodium chloride. The infusion rate of the first infusion was 250 mL/hr; in the absence of infusion reactions (IRs), subsequent infusions were to be administered at an infusion rate of 500 mL/hr. The objective was to evaluate safety in terms of incidence and severity of IRs during the first 2 full 30-min infusions. The initial, recommended premedication to be given at the time of the switch consisted of dexamethasone 20 mg orally (PO) (or equivalent [eq.]), acetaminophen (paracetamol) 650 to 1000 mg PO; ranitidine 50 mg IV (or eq.), diphenhydramine 25 to 50 mg IV (or eq.), and montelukast 10 mg PO (or eq.). Prior to switching, pts received weight-based Isa infusion in the VCd cohorts and initially in the VRd Part A cohort, followed by fixed-volume Isa infusion in both VRd Parts A and B (in Part B, at 200 mL/hr from 3 rd infusion, ~75 min with no IRs/interruptions).
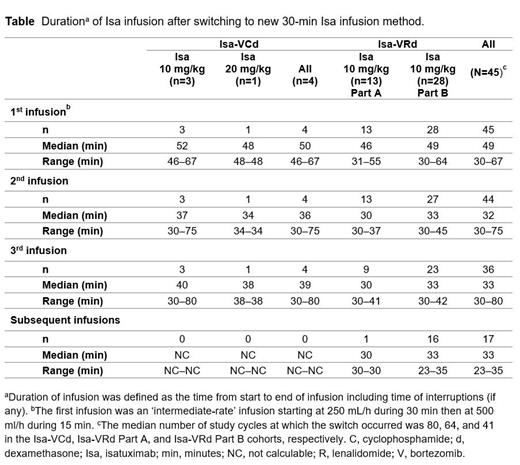
Results: As of 19 May 2023, 29.4% of pts in Isa-VCd, 48.1% in Isa-VRd Part A, and 60.9% in Isa-VRd Part B were still on treatment. The median follow-up for all pts was 71.1, 55.1, and 38.1 months for the Isa-VCd, Isa-VRd Part A, and Isa-VRd Part B cohorts, with a median duration of exposure of 63.5, 54.1, and 40.8 months, respectively. A total of 45 pts received 142 infusions between Jan 2023 and May 2023: 45 first infusions with intermediate rate and 97 30-min infusions across cohorts, with a median of 3 cycles started by pts (range, 1-5) (44 pts received at least 2 infusions) and a median relative Isa dose intensity of 99.4% (range, 73.7-105.5%). Switching occurred at a median of 46 cycles (range, 38-88) for all treated pts. The median duration of Isa infusion in all treated pts was 32 min, 33 min, and 33 min at the 2 nd, 3 rd, and subsequent infusions, respectively ( Table). 30-min infusion of Isa was well tolerated, with no IRs and no infusion interruptions across cohorts.
Conclusions: These preliminary results show that 30-min infusion of Isa is a feasible, well-tolerated, and convenient administration method for pts with multiple myeloma on Isa treatment for several months. The 30-min Isa infusion is currently being assessed from day 1 of cycle 2 in the ongoing Phase 1-2 UMBRELLA trial of Isa with or without dexamethasone in combination with novel agents, conducted in pts with RRMM (NCT04643002).
Clinical trial registration: NCT02513186. Funding: Sanofi.
OffLabel Disclosure:
Ocio:GSK: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Regeneron: Honoraria; Pfizer: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria, Research Funding; Menarini: Consultancy; Karyopharm: Consultancy; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy; Takeda: Consultancy, Honoraria. Perrot:Takeda: Honoraria, Research Funding; Pfizer: Honoraria; Sanofi: Honoraria, Research Funding; AbbVie: Honoraria; Adaptive Biotechnologies: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Janssen: Honoraria. Moreau:janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards; GSK: Honoraria, Other: Advisory Board. Mateos:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Takeda: Honoraria; Regeneron: Honoraria. Bringhen:Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Karlin:AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria; Amgen, Celgene, GSK, Janssen, Takeda: Consultancy. Oprea:Sanofi: Current Employment, Current equity holder in publicly-traded company. Dong:Sanofi: Current Employment, Current equity holder in publicly-traded company. Kodas:Sanofi: Current Employment, Current equity holder in publicly-traded company. San Miguel:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; SecuraBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
30-minute IV administration of isatuximab in combination with bortezomib-cyclophosphamide-dexamethasone or bortezomib-lenalidomide-dexamethasone in patients with newly diagnosed multiple myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal